- Remaining Timing :-
(1). Which is the correct order of increasing energy of the listed orbitals in the atom of titanium ? (At.no.Z=22)
- (a). 4s 3s 3p 3d
- (b). 3s 3p 3d 4s
- (c). 3s 3p 4s 3d
- (d). 3s 4s 3p 3d
- (e). None of these
Answer : 3s 3p 4s 3d
Explanation:
(2). Which of the following pairs are chemically dissimilar ?
- (a). Na and K
- (b). Ba and Sr
- (c). Zr and Hf
- (d). Ca and Zn
- (e). None of these
Answer : Ca and Zn
Explanation:
(3). In the long form of the periodic table, the transition metals are placed in
- (a). s-block
- (b). f-block
- (c). d-block
- (d). s and p-block
- (e). None of these
Answer : d-block
Explanation:
(4). The valency of noble gas, in general, is
- (a). zero
- (b). one
- (c). three
- (d). two
- (e). None of these
Answer : zero
Explanation:
(5). Alkali metals in each period have
- (a). smallest size
- (b). lowest IE
- (c). highest IE
- (d). highest electronegativity
- (e). None of these
Answer : lowest IE
Explanation:
(6). The element with atomic number 55 belongs to which block of the periodic table
- (a). s-block
- (b). p-block
- (c). d-block
- (d). f-block
- (e). None of these
Answer : s-block
Explanation:
(7). 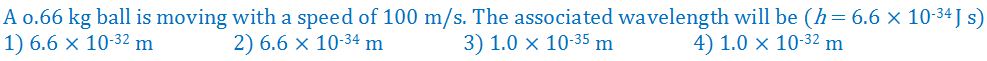
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 3
Explanation:
(8). 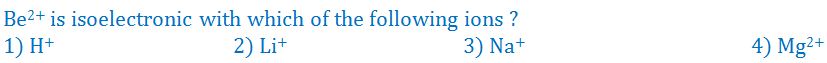
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 2
Explanation:
(9). Which of the following gaseous ions contains maximum number of unpaired electrons ?
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 4
Explanation:
(10). Ionisation energy is highest in
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 2
Explanation: