- Remaining Timing :-
(1). Which one of the alkaline earth metal gives hydrated salts?
- (a). Li
- (b). Na
- (c). K
- (d). Cs
- (e). None of these
Answer : Li
Explanation:
(2). Sodium reacts with water more vigorously than lithium because it
- (a). has highest atomic weight
- (b). is a metal
- (c). is more electronegative
- (d). is more electropositive
- (e). None of these
Answer : is more electropositive
Explanation:
(3). When lime stone is burnt in a kiln the resulting product is
- (a). clinker
- (b). quick lime
- (c). lime mortar
- (d). plaster of paris
- (e). None of these
Answer : quick lime
Explanation:
(4). Alkali metals form hydrated compounds. the hydration enthalpies of alkali metals is in the sequence
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 3
Explanation:
(5). Metals dissolve in liquid ammonia giving coloured solutions which are conducting in nature. the colour of the solution and reason of its conductance is
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 4
Explanation:
(6). Li does not resemble other alkali metals in which of the following property?
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 4
Explanation:
(7). Which of the following imparts violet colouration to the non-luminous flame of bunsen burner?
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 4
Explanation:
(8). Which of the following order is correct for the thermal stability of alkali metal carbonates?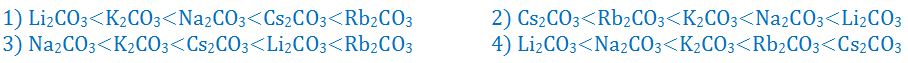
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 4
Explanation:
(9). In solvay ammonia process, sodium bicarbonate is precipitated due to
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 3
Explanation:
(10). Which of the following electronic configurations corresponds to an element with the lowest ionization energy?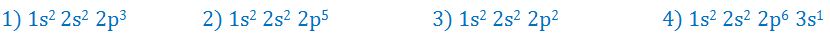
- (a). 1
- (b). 2
- (c). 3
- (d). 4
- (e). None of these
Answer : 4
Explanation: